Quartz
| Quartz | |
|---|---|
 Quartz crystal cluster from Tibet | |
| General | |
| Category | oxide mineral[1][2] |
| Formula (repeating unit) | SiO2 |
| Strunz classification | 4.DA.05 (Oxides) |
| Dana classification | 75.01.03.01 (tectosilicates) |
| Crystal system | α-quartz: trigonal β-quartz: hexagonal |
| Crystal class | α-quartz: trapezohedral (class 3 2); β-quartz: trapezohedral (class 6 2 2)[3] |
| Unit cell | a = 4.9133 Å, c = 5.4053 Å; Z=3 |
| Identification | |
| Formula mass | 60.083 g·mol−1 |
| Color | Colorless through various colors to black |
| Crystal habit | 6-sided prism ending in 6-sided pyramid (typical), drusy, fine-grained to microcrystalline, massive |
| Twinning | Common Dauphine law, Brazil law and Japan law |
| Cleavage | {0110} Indistinct |
| Fracture | Conchoidal |
| Tenacity | Brittle |
| Mohs scale hardness | 7 – lower in impure varieties (defining mineral) |
| Luster | Vitreous – waxy to dull when massive |
| Streak | White |
| Diaphaneity | Transparent to nearly opaque |
| Specific gravity | 2.65; variable 2.59–2.63 in impure varieties |
| Optical properties | Uniaxial (+) |
| Refractive index | nω = 1.543–1.545 nε = 1.552–1.554 |
| Birefringence | +0.009 (B-G interval) |
| Pleochroism | None |
| Melting point | 1670 °C (β tridymite) 1713 °C (β cristobalite)[3] |
| Solubility | Insoluble at STP; 1 ppmmass at 400 °C and 500 lb/in2 to 2600 ppmmass at 500 °C and 1500 lb/in2[3] |
| Other characteristics | lattice: hexagonal, Piezoelectric, may be triboluminescent, chiral (hence optically active if not racemic) |
| References | [1][4][5][6] |
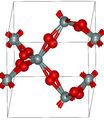
Quartz is a mineral composed of silicon and oxygen atoms in a continuous framework of SiO4 silicon–oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical formula of SiO2. Quartz is the second most abundant mineral in Earth's continental crust, behind feldspar.[7]
Quartz exists in two forms, the normal α-quartz and the high-temperature β-quartz, both of which are chiral. The transformation from α-quartz to β-quartz takes place abruptly at 573 °C (846 K). Since the transformation is accompanied by a significant change in volume, it can easily induce fracturing of ceramics or rocks passing through this temperature threshhold.
There are many different varieties of quartz, several of which are semi-precious gemstones. Since antiquity, varieties of quartz have been the most commonly used minerals in the making of jewelry and hardstone carvings, especially in Eurasia.
Contents
Etymology[edit]
The word "quartz" is derived from the German word "Quarz", which had the same form in the first half of the 14th century in Middle High German in East Central German[8] and which came from the Polish dialect term kwardy, which corresponds to the Czech term tvrdý ("hard").[9]
The Ancient Greeks referred to quartz as κρύσταλλος (krustallos) derived from the Ancient Greek κρύος (kruos) meaning "icy cold", because some philosophers (including Theophrastus) apparently believed the mineral to be a form of supercooled ice.[10] Today, the term rock crystal is sometimes used as an alternative name for the purest form of quartz.
Crystal habit and structure[edit]

Quartz belongs to the trigonal crystal system. The ideal crystal shape is a six-sided prism terminating with six-sided pyramids at each end. In nature quartz crystals are often twinned (with twin right-handed and left-handed quartz crystals), distorted, or so intergrown with adjacent crystals of quartz or other minerals as to only show part of this shape, or to lack obvious crystal faces altogether and appear massive. Well-formed crystals typically form in a 'bed' that has unconstrained growth into a void; usually the crystals are attached at the other end to a matrix and only one termination pyramid is present. However, doubly terminated crystals do occur where they develop freely without attachment, for instance within gypsum. A quartz geode is such a situation where the void is approximately spherical in shape, lined with a bed of crystals pointing inward.
α-quartz crystallizes in the trigonal crystal system, space group P3121 or P3221 depending on the chirality. β-quartz belongs to the hexagonal system, space group P6222 and P6422, respectively.[11] These space groups are truly chiral (they each belong to the 11 enantiomorphous pairs). Both α-quartz and β-quartz are examples of chiral crystal structures composed of achiral building blocks (SiO4 tetrahedra in the present case). The transformation between α- and β-quartz only involves a comparatively minor rotation of the tetrahedra with respect to one another, without change in the way they are linked.
Varieties (according to microstructure)[edit]
Although many of the varietal names historically arose from the color of the mineral, current scientific naming schemes refer primarily to the microstructure of the mineral. Color is a secondary identifier for the cryptocrystalline minerals, although it is a primary identifier for the macrocrystalline varieties.[12]
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Varieties (according to color)[edit]

Pure quartz, traditionally called rock crystal or clear quartz, is colorless and transparent or translucent, and has often been used for hardstone carvings, such as the Lothair Crystal. Common colored varieties include citrine, rose quartz, amethyst, smoky quartz, milky quartz, and others.[13]
The most important distinction between types of quartz is that of macrocrystalline (individual crystals visible to the unaided eye) and the microcrystalline or cryptocrystalline varieties (aggregates of crystals visible only under high magnification). The cryptocrystalline varieties are either translucent or mostly opaque, while the transparent varieties tend to be macrocrystalline. Chalcedony is a cryptocrystalline form of silica consisting of fine intergrowths of both quartz, and its monoclinic polymorph moganite.[14] Other opaque gemstone varieties of quartz, or mixed rocks including quartz, often including contrasting bands or patterns of color, are agate, carnelian or sard, onyx, heliotrope, and jasper.
Amethyst[edit]
Amethyst is a form of quartz that ranges from a bright to dark or dull purple color. The world's largest deposits of amethysts can be found in Brazil, Mexico, Uruguay, Russia, France, Namibia and Morocco. Sometimes amethyst and citrine are found growing in the same crystal. It is then referred to as ametrine. An amethyst is formed when there is iron in the area where it was formed.
Blue quartz[edit]
Blue quartz contains inclusions of fibrous magnesio-riebeckite or crocidolite.[15]
Dumortierite quartz[edit]
Inclusions of the mineral dumortierite within quartz pieces often result in silky-appearing splotches with a blue hue, shades giving off purple and/or grey colors additionally being found. "Dumortierite quartz" (sometimes called "blue quartz") will sometimes feature contrasting light and dark color zones across the material.[16][17] Interest in the certain quality forms of blue quartz as a collectible gemstone particularly arises in India and in the United States.[16]
Citrine[edit]
Citrine is a variety of quartz whose color ranges from a pale yellow to brown due to ferric impurities. Natural citrines are rare; most commercial citrines are heat-treated amethysts or smoky quartzes. However, a heat-treated amethyst will have small lines in the crystal, as opposed to a natural citrine's cloudy or smokey appearance. It is nearly impossible to differentiate between cut citrine and yellow topaz visually, but they differ in hardness. Brazil is the leading producer of citrine, with much of its production coming from the state of Rio Grande do Sul. The name is derived from the Latin word citrina which means "yellow" and is also the origin of the word "citron". Sometimes citrine and amethyst can be found together in the same crystal, which is then referred to as ametrine.[18] Citrine has been referred to as the "merchant's stone" or "money stone", due to a superstition that it would bring prosperity.[19]
Citrine was first appreciated as a golden-yellow gemstone in Greece between 300 and 150 BC, during the Hellenistic Age. The yellow quartz was used prior to that to decorate jewelry and tools but it was not highly sought-after.[20]
Milky quartz[edit]
Milk quartz or milky quartz is the most common variety of crystalline quartz. The white color is caused by minute fluid inclusions of gas, liquid, or both, trapped during crystal formation,[21] making it of little value for optical and quality gemstone applications.[22]
Rose quartz[edit]
Rose quartz is a type of quartz which exhibits a pale pink to rose red hue. The color is usually considered as due to trace amounts of titanium, iron, or manganese, in the material. Some rose quartz contains microscopic rutile needles which produces an asterism in transmitted light. Recent X-ray diffraction studies suggest that the color is due to thin microscopic fibers of possibly dumortierite within the quartz.[23]
Additionally, there is a rare type of pink quartz (also frequently called crystalline rose quartz) with color that is thought to be caused by trace amounts of phosphate or aluminium. The color in crystals is apparently photosensitive and subject to fading. The first crystals were found in a pegmatite found near Rumford, Maine, USA and in Minas Gerais, Brazil.[24]
Smoky quartz[edit]
Smoky quartz is a gray, translucent version of quartz. It ranges in clarity from almost complete transparency to a brownish-gray crystal that is almost opaque. Some can also be black. The translucency results from natural irradiation creating free silicon within the crystal.
Prasiolite[edit]
Prasiolite, also known as vermarine, is a variety of quartz that is green in color. Since 1950, almost all natural prasiolite has come from a small Brazilian mine, but it is also seen in Lower Silesia in Poland. Naturally occurring prasiolite is also found in the Thunder Bay area of Canada. It is a rare mineral in nature; most green quartz is heat-treated amethyst.[25]
Synthetic and artificial treatments[edit]

Not all varieties of quartz are naturally occurring. Some clear quartz crystals can be treated using heat or gamma-irradiation to induce color where it would not otherwise have occurred naturally. Susceptibility to such treatments depends on the location from which the quartz was mined.[26]
Prasiolite, an olive colored material, is produced by heat treatment; natural prasiolite has also been observed in Lower Silesia in Poland. Although citrine occurs naturally, the majority is the result of heat-treating amethyst or smoky quartz. Carnelian is widely heat-treated to deepen its color.
Because natural quartz is often twinned, synthetic quartz is produced for use in industry. Large, flawless, single crystals are synthesized in an autoclave via the hydrothermal process; emeralds are also synthesized in this fashion.
Like other crystals, quartz may be coated with metal vapors to give it an attractive sheen.
Occurrence[edit]

Quartz is a defining constituent of granite and other felsic igneous rocks. It is very common in sedimentary rocks such as sandstone and shale. It is a common constituent of schist, gneiss, quartzite and other metamorphic rocks. Quartz has the lowest potential for weathering in the Goldich dissolution series and consequently it is very common as a residual mineral in stream sediments and residual soils.
While the majority of quartz crystallizes from molten magma, much quartz also chemically precipitates from hot hydrothermal veins as gangue, sometimes with ore minerals like gold, silver and copper. Large crystals of quartz are found in magmatic pegmatites. Well-formed crystals may reach several meters in length and weigh hundreds of kilograms.
Naturally occurring quartz crystals of extremely high purity, necessary for the crucibles and other equipment used for growing silicon wafers in the semiconductor industry, are expensive and rare. A major mining location for high purity quartz is the Spruce Pine Gem Mine in Spruce Pine, North Carolina, United States.[27] Quartz may also be found in Caldoveiro Peak, in Asturias, Spain.[28]
The largest documented single crystal of quartz was found near Itapore, Goiaz, Brazil; it measured approximately 6.1×1.5×1.5 m and weighed more than 44 tonnes.[29]
Related silica minerals[edit]
Tridymite and cristobalite are high-temperature polymorphs of SiO2 that occur in high-silica volcanic rocks. Coesite is a denser polymorph of SiO2 found in some meteorite impact sites and in metamorphic rocks formed at pressures greater than those typical of the Earth's crust. Stishovite is a yet denser and higher-pressure polymorph of SiO2 found in some meteorite impact sites. Lechatelierite is an amorphous silica glass SiO2 which is formed by lightning strikes in quartz sand.
History[edit]

The word "quartz" comes from the German ![]() Quarz (help·info),[30] which is of Slavic origin (Czech miners called it křemen). Other sources attribute the word's origin to the Saxon word Querkluftertz, meaning cross-vein ore.[31]
Quarz (help·info),[30] which is of Slavic origin (Czech miners called it křemen). Other sources attribute the word's origin to the Saxon word Querkluftertz, meaning cross-vein ore.[31]
Quartz is the most common material identified as the mystical substance maban in Australian Aboriginal mythology. It is found regularly in passage tomb cemeteries in Europe in a burial context, such as Newgrange or Carrowmore in Ireland. The Irish word for quartz is grianchloch, which means 'sunstone'. Quartz was also used in Prehistoric Ireland, as well as many other countries, for stone tools; both vein quartz and rock crystal were knapped as part of the lithic technology of the prehistoric peoples.[32]
While jade has been since earliest times the most prized semi-precious stone for carving in East Asia and Pre-Columbian America, in Europe and the Middle East the different varieties of quartz were the most commonly used for the various types of jewelry and hardstone carving, including engraved gems and cameo gems, rock crystal vases, and extravagant vessels. The tradition continued to produce objects that were very highly valued until the mid-19th century, when it largely fell from fashion except in jewelry. Cameo technique exploits the bands of color in onyx and other varieties.

Roman naturalist Pliny the Elder believed quartz to be water ice, permanently frozen after great lengths of time.[34] (The word "crystal" comes from the Greek word κρύσταλλος, "ice".) He supported this idea by saying that quartz is found near glaciers in the Alps, but not on volcanic mountains, and that large quartz crystals were fashioned into spheres to cool the hands. This idea persisted until at least the 17th century. He also knew of the ability of quartz to split light into a spectrum.
In the 17th century, Nicolas Steno's study of quartz paved the way for modern crystallography. He discovered that regardless of a quartz crystal's size or shape, its long prism faces always joined at a perfect 60° angle.[35]
Quartz's piezoelectric properties were discovered by Jacques and Pierre Curie in 1880.[36][37] The quartz oscillator or resonator was first developed by Walter Guyton Cady in 1921.[38][39] George Washington Pierce designed and patented quartz crystal oscillators in 1923.[40][41][42] Warren Marrison created the first quartz oscillator clock based on the work of Cady and Pierce in 1927.[43]
Efforts to synthesize quartz began in the mid nineteenth century as scientists attempted to create minerals under laboratory conditions that mimicked the conditions in which the minerals formed in nature: German geologist Karl Emil von Schafhäutl (1803–1890)[44] was the first person to synthesize quartz when in 1845 he created microscopic quartz crystals in a pressure cooker.[45] However, the quality and size of the crystals that were produced by these early efforts were poor.[46]

By the 1930s, the electronics industry had become dependent on quartz crystals. The only source of suitable crystals was Brazil; however, World War II disrupted the supplies from Brazil, so nations attempted to synthesize quartz on a commercial scale. German mineralogist Richard Nacken (1884–1971) achieved some success during the 1930s and 1940s.[47] After the war, many laboratories attempted to grow large quartz crystals. In the United States, the U.S. Army Signal Corps contracted with Bell Laboratories and with the Brush Development Company of Cleveland, Ohio to synthesize crystals following Nacken's lead.[48][49] (Prior to World War II, Brush Development produced piezoelectric crystals for record players.) By 1948, Brush Development had grown crystals that were 1.5 inches (3.8 cm) in diameter, the largest to date.[50][51] By the 1950s, hydrothermal synthesis techniques were producing synthetic quartz crystals on an industrial scale, and today virtually all the quartz crystal used in the modern electronics industry is synthetic.
Piezoelectricity[edit]
Some types of quartz crystals have piezoelectric properties; they develop an electric potential upon the application of mechanical stress.[52] An early use of this property of quartz crystals was in phonograph pickups. One of the most common piezoelectric uses of quartz today is as a crystal oscillator. The quartz clock is a familiar device using the mineral. The resonant frequency of a quartz crystal oscillator is changed by mechanically loading it, and this principle is used for very accurate measurements of very small mass changes in the quartz crystal microbalance and in thin-film thickness monitors.
See also[edit]
References[edit]
- ^ a b Quartz Archived 14 December 2005 at the Wayback Machine. Mindat.org. Retrieved 2013-03-07.
- ^ Quartz page on Mineralien Atlas
- ^ a b c Deer, W. A., R. A. Howie and J. Zussman, An Introduction to the Rock Forming Minerals, Logman, 1966, pp. 340–355 ISBN 0-582-44210-9
- ^ Anthony, John W.; Bideaux, Richard A.; Bladh, Kenneth W.; Nichols, Monte C. (eds.). "Quartz". Handbook of Mineralogy (PDF). III (Halides, Hydroxides, Oxides). Chantilly, VA, US: Mineralogical Society of America. ISBN 0962209724. Archived (PDF) from the original on 1 April 2010. Retrieved 21 October 2009.
- ^ Quartz Archived 12 November 2006 at the Wayback Machine. Webmineral.com. Retrieved 2013-03-07.
- ^ Hurlbut, Cornelius S.; Klein, Cornelis (1985). Manual of Mineralogy (20 ed.). ISBN 0-471-80580-7.
- ^ Anderson, Robert S.; Anderson, Suzanne P. (2010). Geomorphology: The Mechanics and Chemistry of Landscapes. Cambridge University Press. p. 187. ISBN 978-1-139-78870-0.
- ^ Digitales Wörterbuch der deutschen Sprache Archived 1 December 2017 at the Wayback Machine (in German)
- ^ "{title}". Archived from the original on 1 December 2017. Retrieved 26 November 2017.
- ^ Tomkeieff, S.I. (1942). "On the origin of the name 'quartz'" (PDF). Mineralogical Magazine. 26: 172–178. doi:10.1180/minmag.1942.026.176.04. Archived (PDF) from the original on 4 September 2015. Retrieved 12 August 2015.
- ^ Crystal Data, Determinative Tables, ACA Monograph No. 5, American Crystallographic Association, 1963
- ^ "Quartz Gemstone and Jewelry Information: Natural Quartz - GemSelect". www.gemselect.com. Archived from the original on 29 August 2017. Retrieved 29 August 2017.
- ^ "Quartz: The gemstone Quartz information and pictures". www.minerals.net. Archived from the original on 27 August 2017. Retrieved 29 August 2017.
- ^ Heaney, Peter J. (1994). "Structure and Chemistry of the low-pressure silica polymorphs". Reviews in Mineralogy and Geochemistry. 29 (1): 1–40. Archived from the original on 24 July 2011. Retrieved 26 October 2009.
- ^ "Blue Quartz". Mindat.org. Archived from the original on 24 February 2017. Retrieved 24 February 2017.
- ^ a b Oldershaw, Cally (2003). Firefly Guide to Gems. Firefly Books. p. 100. ISBN 9781552978146. Archived from the original on 22 February 2017. Retrieved 19 February 2017.
- ^ "The Gemstone Dumortierite". Minerals.net. Archived from the original on 6 May 2017. Retrieved 23 April 2017.
- ^ Citrine Archived 2 May 2010 at the Wayback Machine. Mindat.org (2013-03-01). Retrieved 2013-03-07.
- ^ The Encyclopedia of Superstitions By Richard Webster, p.19
- ^ "{title}". Archived from the original on 18 August 2017. Retrieved 18 August 2017.
- ^ Hurrell, Karen; Johnson, Mary L. (2016-12-15). Gemstones: A Complete Color Reference for Precious and Semiprecious Stones of the World. Book Sales. p. 97. ISBN 978-0-7858-3498-4.
- ^ Milky quartz at Mineral Galleries Archived 19 December 2008 at the Wayback Machine. Galleries.com. Retrieved 2013-03-07.
- ^ Rose Quartz Archived 1 April 2009 at the Wayback Machine. Mindat.org (2013-02-18). Retrieved 2013-03-07.
- ^ Colored Varieties of Quartz Archived 19 July 2011 at the Wayback Machine, Caltech
- ^ "Prasiolite". quarzpage.de. 28 October 2009. Archived from the original on 13 July 2011. Retrieved 28 November 2010.
- ^ Liccini, Mark, Treating Quartz to Create Color Archived 23 December 2014 at the Wayback Machine, International Gem Society website. Retrieved 22 December 2014
- ^ Nelson, Sue (2 August 2009). "Silicon Valley's secret recipe". BBC News. Archived from the original on 5 August 2009. Retrieved 16 September 2009.
- ^ "Caldoveiro Mine, Tameza, Asturias, Spain". mindat.org. Archived from the original on 12 February 2018. Retrieved 15 February 2018.
- ^ Rickwood, P. C. (1981). "The largest crystals" (PDF). American Mineralogist. 66: 885–907 (903). Archived (PDF) from the original on 25 August 2013. Retrieved 7 March 2013.
- ^ German Loan Words in English Archived 21 August 2007 at the Wayback Machine. German.about.com (2012-04-10). Retrieved 2013-03-07.
- ^ Mineral Atlas Archived 4 September 2007 at the Wayback Machine, Queensland University of Technology. Mineralatlas.com. Retrieved 2013-03-07.
- ^ "Driscoll, Killian. 2010. Understanding quartz technology in early prehistoric Ireland". Archived from the original on 25 June 2017. Retrieved 19 July 2017.
- ^ The International Antiques Yearbook. Studio Vista Limited. 1972. p. 78.
Apart from Prague and Florence, the main Renaissance centre for crystal cutting was Milan.
- ^ Pliny the Elder, The Natural History, Book 37, Chapter 9. Available on-line at: Perseus.Tufts.edu Archived 9 November 2012 at the Wayback Machine.
- ^ Nicolaus Steno (Latinized name of Niels Steensen) with John Garrett Winter, trans., The Prodromus of Nicolaus Steno's Dissertation Concerning a Solid Body Enclosed by Process of Nature Within a Solid (New York, New York: Macmillan Co., 1916). On page 272 Archived 4 September 2015 at the Wayback Machine, Steno states his law of constancy of interfacial angles: "Figures 5 and 6 belong to the class of those which I could present in countless numbers to prove that in the plane of the axis both the number and the length of the sides are changed in various ways without changing the angles; … "
- ^ Curie, Jacques; Curie, Pierre (1880). "Développement par compression de l'électricité polaire dans les cristaux hémièdres à faces inclinées" [Development, via compression, of electric polarization in hemihedral crystals with inclined faces]. Bulletin de la Société minérologique de France. 3: 90–93.. Reprinted in: Curie, Jacques; Curie, Pierre (1880). "Développement, par pression, de l'électricité polaire dans les cristaux hémièdres à faces inclinées". Comptes rendus. 91: 294–295. Archived from the original on 5 December 2012. Retrieved 17 December 2013.
- ^ Curie, Jacques; Curie, Pierre (1880). "Sur l'électricité polaire dans les cristaux hémièdres à faces inclinées" [On electric polarization in hemihedral crystals with inclined faces]. Comptes rendus. 91: 383–386. Archived from the original on 5 December 2012. Retrieved 17 December 2013.
- ^ Cady, W. G. (1921). "The piezoelectric resonator". Physical Review. 17: 531–533. doi:10.1103/PhysRev.17.508.
- ^ "The Quartz Watch – Walter Guyton Cady". The Lemelson Center, National Museum of American History. Smithsonian Institution. Archived from the original on 4 January 2009.
- ^ Pierce, G. W. (1923). "Piezoelectric crystal resonators and crystal oscillators applied to the precision calibration of wavemeters". Proceedings of the American Academy of Arts and Sciences. 59 (4): 81–106. doi:10.2307/20026061. JSTOR 20026061.
- ^ Pierce, George W. "Electrical system," U.S. Patent 2,133,642, filed: 25 February 1924; issued: 18 October 1938.
- ^ "The Quartz Watch – George Washington Pierce". The Lemelson Center, National Museum of American History. Smithsonian Institution. Archived from the original on 4 January 2009.
- ^ "The Quartz Watch – Warren Marrison". The Lemelson Center, National Museum of American History. Smithsonian Institution. Archived from the original on 25 January 2009.
- ^ For biographical information about Karl von Schafhäutl, see German Wikipedia's article: Karl Emil von Schafhäutl (in German).
- ^ von Schafhäutl, Karl Emil (10 April 1845). "Die neuesten geologischen Hypothesen und ihr Verhältniß zur Naturwissenschaft überhaupt (Fortsetzung)" [The latest geological hypotheses and their relation to science in general (continuation)]. Gelehrte Anzeigen. München: im Verlage der königlichen Akademie der Wissenschaften, in Commission der Franz'schen Buchhandlung. 20 (72): 577–584. OCLC 1478717. From page 578: 5) Bildeten sich aus Wasser, in welchen ich im Papinianischen Topfe frisch gefällte Kieselsäure aufgelöst hatte, beym Verdampfen schon nach 8 Tagen Krystalle, die zwar mikroscopisch, aber sehr wohl erkenntlich aus sechseitigen Prismen mit derselben gewöhnlichen Pyramide bestanden. ( 5) There formed from water in which I had dissolved freshly precipitated silicic acid in a Papin pot [i.e., pressure cooker], after just 8 days of evaporating, crystals, which albeit were microscopic but consisted of very easily recognizable six-sided prisms with their usual pyramids.)
- ^ Byrappa, K. and Yoshimura, Masahiro (2001) Handbook of Hydrothermal Technology. Norwich, New York: Noyes Publications. ISBN 008094681X. Chapter 2: History of Hydrothermal Technology.
- ^ Nacken, R. (1950) "Hydrothermal Synthese als Grundlage für Züchtung von Quarz-Kristallen" (Hydrothermal synthesis as a basis for the production of quartz crystals), Chemiker Zeitung, 74 : 745–749.
- ^ Hale, D. R. (1948). "The Laboratory Growing of Quartz". Science. 107 (2781): 393–394. doi:10.1126/science.107.2781.393.
- ^ Lombardi, M. (2011). "The evolution of time measurement, Part 2: Quartz clocks [Recalibration]" (PDF). IEEE Instrumentation & Measurement Magazine. 14 (5): 41–48. doi:10.1109/MIM.2011.6041381. Archived (PDF) from the original on 27 May 2013. Retrieved 30 March 2013.
- ^ "Record crystal," Popular Science, 154 (2) : 148 (February 1949).
- ^ Brush Development's team of scientists included: Danforth R. Hale, Andrew R. Sobek, and Charles Baldwin Sawyer (1895–1964). The company's U.S. patents included:
- Sobek, Andrew R. "Apparatus for growing single crystals of quartz," U.S. Patent 2,674,520; filed: 11 April 1950; issued: 6 April 1954.
- Sobek, Andrew R. and Hale, Danforth R. "Method and apparatus for growing single crystals of quartz," U.S. Patent 2,675,303; filed: 11 April 1950; issued: 13 April 1954.
- Sawyer, Charles B. "Production of artificial crystals," U.S. Patent 3,013,867; filed: 27 March 1959; issued: 19 December 1961. (This patent was assigned to Sawyer Research Products of Eastlake, Ohio.)
- ^ Forwood, Anthony K. (2011). They Would Be Gods. Lulu.com. p. 302. ISBN 978-1-257-37362-8.
External links[edit]
| Wikimedia Commons has media related to Quartz. |
| Wikisource has original text related to this article: |
- Quartz varieties, properties, crystal morphology. Photos and illustrations
- Gilbert Hart, "Nomenclature of Silica", American Mineralogist, Volume 12, pages 383–395, 1927
- "The Quartz Watch – Inventors". The Lemelson Center, National Museum of American History. Smithsonian Institution. Archived from the original on 7 January 2009.
- Terminology used to describe the characteristics of quartz crystals when used as oscillators
- Quartz use as prehistoric stone tool raw material




_Can_you_imagine%3F_(5320329773).jpg/133px-(61-365)_Can_you_imagine%3F_(5320329773).jpg)


.jpg/133px-Citrine_1_(Russie).jpg)




