Ritonavir
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Norvir |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a696029 |
| License data | |
| Pregnancy category | |
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 98-99% |
| Metabolism | Hepatic |
| Elimination half-life | 3-5 hours |
| Excretion | mostly fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| ECHA InfoCard | 100.125.710 |
| Chemical and physical data | |
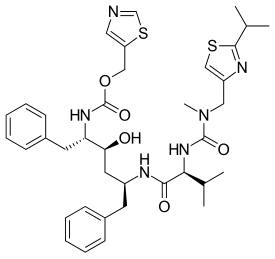
| Formula | C37H48N6O5S2 |
| Molar mass | 720.946 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ritonavir, sold under the trade name Norvir, is an antiretroviral medication used along with other medications to treat HIV/AIDS.[1] This combination treatment is known as highly active antiretroviral therapy (HAART).[1] Often a low dose is used with other protease inhibitors.[1] It may also be used in combination with other medications for hepatitis C.[2] It is taken by mouth.[1] The capsules of the medication do not work the same as the tablets.[1]
Common side effects include nausea, vomiting, loss of appetite, diarrhea, and numbness of the hands and feet.[1] Serious side effects include liver problems, pancreatitis, allergic reactions, and arrythmias.[1] Serious interactions may occur with a number of other medications including amiodarone and simvastatin.[1] At low doses it is considered to be acceptable for use during pregnancy.[3] Ritonavir is of the protease inhibitor class.[1] It is often used to inhibit the enzyme that metabolizes other protease inhibitors.[4] This inhibition leads to higher concentrations of these latter medication.[4]
Ritonavir first came into use in 1996.[5] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[6] Globally the wholesale cost in the developing world is between US$0.07 and $2.20 per day.[7] In the United States it costs about $9.20–55 per day depending on the dose.[1]
Contents
Medical uses[edit]
Ritonavir is used along with other medications to treat HIV/AIDS.[1]
Side effects[edit]
When administered at doses effective for anti-HIV therapy, the side effects of ritonavir are those shown below. It is currently (2015) much more widely used at lower doses as a pharmacokinetic inhibitor. The adverse effects of these lower doses of ritonavir do not appear to have been extensively characterized.[8]
- asthenia, malaise
- diarrhea
- nausea and vomiting
- abdominal pain
- dizziness
- insomnia
- sweating
- taste abnormality
- metabolic
- hypercholesterolemia
- hypertriglyceridemia
- elevated transaminases
- elevated CPK
One of ritonavir's side effects is hyperglycemia. It appears that ritonavir directly inhibits the GLUT4 insulin-regulated transporter, keeping glucose from entering fat and muscle cells.[citation needed] This can lead to insulin resistance and cause problems for people with type Ⅱ diabetes. The capsules of the medication do not have the same bioavailability as the tablets.[1]
Drug interactions[edit]
Concomitant therapy of ritonavir with a variety of medications may result in serious and sometimes fatal drug interactions.[9] Ritonavir induces CYP 1A2 and inhibits the major P450 isoforms (3A4 and 2D6).
The list of clinically significant interactions of ritonavir includes but is not limited to the following drugs:
- amiodarone—decreased metabolism, possible toxicity
- bosentan—decreased metabolism via CYP3A4, stop bosentan 36 hours prior to start ritonavir, slow resume
- midazolam and triazolam—contraindicated
- carbamazepine—decreased metabolism, possible toxicity
- cisapride—decreased metabolism, possible prolongation of Q-T interval and life-threatening arrythmias
- disulfiram (with ritonavir oral preparation)—decreased metabolism of ritonavir
- eplerenone
- etravirine
- flecainide—decreased metabolism, possible toxicity
- MDMA—decreased metabolism, can sometimes result in toxic outcomes such as serotonin syndrome, which can be life-threatening[10][11]
- mescaline
- meperidine (pethidine)—build-up of toxic concentrations of norpethidine possible
- nilotinib
- nisoldipine
- oxycodone— greatly increased concentrations of oxycodone[12]
- phenytoin—the proposed mechanism involves ritonavir induction of phenytoin metabolism via CYP450 2C9
- pimozide
- quinidine
- ranolazine
- salmeterol
- saquinavir— ritonavir inhibits its metabolism, but the bioavailability can decrease due to in vivo supersaturation[13]
- St John's wort
- statins—decreased metabolism, without dosage modification increased risk of rhabdomyolysis
- thioridazine
- topotecan
- voriconazole—ritonavir increases metabolism of voriconazole
Mechanism of action[edit]
Ritonavir was originally developed as an inhibitor of HIV protease. It is one of the most complex inhibitors. It is now rarely used for its own antiviral activity, but remains widely used as a booster of other protease inhibitors. More specifically, ritonavir is used to inhibit a particular liver enzyme that normally metabolizes protease inhibitors, cytochrome P450-3A4 (CYP3A4).[14] The drug's molecular structure inhibits CYP3A4, so a low dose can be used to enhance other protease inhibitors. This discovery, which has drastically reduced the adverse effects and improved the efficacy of protease inhibitors and HAART, was first communicated in an article published in the journal AIDS in 1997 by researchers at the University of Liverpool.[15] This effect does come with a price: it also affects the efficacy of numerous other medications, making it difficult to know how to administer them concurrently.
History[edit]

Ritonavir is manufactured as Norvir by AbbVie, Inc.. The Food and Drug Administration (FDA) approved ritonavir on March 1, 1996, making it the seventh approved antiretroviral drug and the second approved protease inhibitor in the United States. Within 2 years of the approval of ritonavir (and of saquinavir a few months earlier), the U.S. HIV-associated death rate fell from over 50,000 per year to about 18,000.[17]
In 2003, Abbott (now AbbVie, Inc.) raised the price of a Norvir course from USD $1.71 per day to $8.57 per day, leading to claims of price gouging by patients' groups and some members of Congress. Consumer group Essential Inventions petitioned the NIH to override the Norvir patent, but the NIH announced on August 4, 2004 that it lacked the legal right to allow generic production of Norvir.[18]
In 2014, FDA approved a combination of ombitasvir/paritaprevir/ritonavir for treatment hepatitis C virus (HCV) genotype 4.[2]
Polymorphism and temporary market withdrawal[edit]
Ritonavir was originally dispensed as an ordinary capsule, which did not require refrigeration. This was as a crystal of what is now called form I.[19] However, like many drugs, ritonavir exhibits polymorphism, i.e., the same molecule crystallizes into more than one type of crystal. The different crystals, or polymorphs, are made of the same molecules but in different crystalline arrangements. The solubility and hence the bioavailability is very different in the two different arrangements.[20]
During development (it was introduced in 1996), only the polymorph now called form I was found, but in 1998, a lower free energy, more stable polymorph (form II) appeared. This more stable (and so less soluble) crystal form compromised the oral bioavailability of the drug. This caused the removal of the oral capsule formulation from the market.[20]
Even a trace of form II can catalyse the transformation from the more bioavailable form I to form II. Thus form II threatened existing supplies of ritonavir as the lower solubility polymorph caused the therapeutically effective polymorph to convert to form II. Form II, which was not therapeutically effective because of poor solubility and resulting much lower bioavailability, entered production lines and effectively halted production processes.[19]
After this discovery in the late 1990s, Abbott (now AbbVie) withdrew the original capsules from the market, and recommended people switch to Norvir suspension while researchers worked to solve the problem. The capsules have been replaced with refrigerated gelcaps, to solve the crystallization problem of the original capsules.
In 2000 Abbott (now AbbVie) was awarded approval by the FDA for a tablet (called lopinavir/ritonavir) which contains ritonavir that does not require refrigeration.[21]
References[edit]
- ^ a b c d e f g h i j k l "Ritonavir". The American Society of Health-System Pharmacists. Archived from the original on 2015-10-17. Retrieved Oct 23, 2015.
- ^ a b "FDA approves Viekira Pak to treat hepatitis C". Food and Drug Administration. December 19, 2014. Archived from the original on October 31, 2015.
- ^ "Ritonavir Pregnancy and Breastfeeding Warnings". drugs.com. Archived from the original on 7 September 2015. Retrieved 23 October 2015.
- ^ a b British National Formulary 69 (69 ed.). Pharmaceutical Pr. March 31, 2015. p. 426. ISBN 9780857111562.
- ^ Hacker, Miles (2009). Pharmacology principles and practice. Amsterdam: Academic Press/Elsevier. p. 550. ISBN 9780080919225.
- ^ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Archived (PDF) from the original on 13 December 2016. Retrieved 8 December 2016.
- ^ "Ritonavir". International Drug Price Indicator Guide. Archived from the original on 10 May 2017. Retrieved 23 October 2015.
- ^ Norvir Archived 2007-06-27 at the Wayback Machine, rxlist.com
- ^ Ritonavir Archived 2008-04-30 at the Wayback Machine, Merck Manual
- ^ Henry, J. A.; Hill, I. R. (1998). "Fatal interaction between ritonavir and MDMA". Lancet. 352 (9142): 1751–1752. doi:10.1016/s0140-6736(05)79824-x. PMID 9848354.
- ^ Papaseit, E.; Vázquez, A.; Pérez-Mañá, C.; Pujadas, M.; De La Torre, R.; Farré, M.; Nolla, J. (2012). "Surviving life-threatening MDMA (3,4-methylenedioxymethamphetamine, ecstasy) toxicity caused by ritonavir (RTV)". Intensive Care Medicine. 38 (7): 1239–1240. doi:10.1007/s00134-012-2537-9. PMID 22460853.
- ^ Nieminen, Tuija H.; Hagelberg, Nora M.; Saari, Teijo I.; Neuvonen, Mikko; Neuvonen, Pertti J.; Laine, Kari; Olkkola, Klaus T. (2010). "Oxycodone concentrations are greatly increased by the concomitant use of ritonavir or lopinavir/ritonavir". European Journal of Clinical Pharmacology. 66 (10): 977–985. doi:10.1007/s00228-010-0879-1. ISSN 0031-6970.
- ^ Hsieh, Yi-Ling; Ilevbare, Grace A.; Van Eerdenbrugh, Bernard; Box, Karl J.; Sanchez-Felix, Manuel Vincente; Taylor, Lynne S. (2012-05-12). "pH-Induced Precipitation Behavior of Weakly Basic Compounds: Determination of Extent and Duration of Supersaturation Using Potentiometric Titration and Correlation to Solid State Properties". Pharmaceutical Research. 29 (10): 2738–2753. doi:10.1007/s11095-012-0759-8. ISSN 0724-8741.
- ^ Zeldin RK, Petruschke RA (2004). "Pharmacological and therapeutic properties of ritonavir-boosted protease inhibitor therapy in HIV-infected patients". Journal of Antimicrobial Chemotherapy. 53 (1): 4–9. doi:10.1093/jac/dkh029. PMID 14657084. Archived from the original on 2007-08-21.
- ^ Merry, Concepta; Barry, Michael G.; Mulcahy, Fiona; Ryan, Mairin; Heavey, Jane; Tjia, John F.; Gibbons, Sara E.; Breckenridge, Alasdair M.; Back, David J. (1997). "Saquinavir pharmacokinetics alone and in combination with ritonavir in HIV-infected patients". AIDS. 11 (4): F29–F33. doi:10.1097/00002030-199704000-00001. PMID 9084785.
- ^ "www.cdc.gov" (PDF). Archived (PDF) from the original on 2015-09-24.
- ^ "HIV Surveillance --- United States, 1981--2008". Archived from the original on 9 November 2013. Retrieved 8 November 2013.
- ^ Ceci Connolly (2004-08-05). "NIH Declines to Enter AIDS Drug Price Battle". Washington Post. Archived from the original on 2008-08-20. Retrieved 2006-01-16.
- ^ a b Bauer J, et al. (2001). "Ritonavir: An Extraordinary Example of Conformational Polymorphism". Pharmaceutical Research. 18 (6): 859–866. doi:10.1023/A:1011052932607. PMID 11474792.
- ^ a b S. L. Morissette; S. Soukasene; D. Levinson; M. J. Cima; O. Almarsson (2003). "Elucidation of crystal form diversity of the HIV protease inhibitor ritonavir by high-throughput crystallization". Proc. Natl. Acad. Sci. USA. 100 (5): 2180–84. doi:10.1073/pnas.0437744100. PMC 151315. PMID 12604798. Archived from the original on 2016-09-29.
- ^ "KALETRA FAQ". AbbVie's Kaletra product information. AbbVie. 2011. Archived from the original on 7 July 2014. Retrieved 5 July 2014.
Further reading[edit]
- Chemburkar, Sanjay R.; Bauer, John; Deming, Kris; Spiwek, Harry; Patel, Ketan; Morris, John; Henry, Rodger; Spanton, Stephen; et al. (2000). "Dealing with the Impact of Ritonavir Polymorphs on the Late Stages of Bulk Drug Process Development". Organic Process Research & Development. 4 (5): 413. doi:10.1021/op000023y.
External links[edit]
- PubPK - Ritonavir pharmacokinetics
- [1] (manufacturer's website)
- Ritonavir bound to proteins in the PDB
- Norvir Prescribing Information
